Treatment of Sulfur Plant Effluent to Avoid Atmospheric Pollution (Patent No: 938087)
Inventor: Caruther, Neal M. Location: Tulsa, OK Comments: N/A Description: THE EMBODIMENTS OF THE INVENTION IN WHICH AN EXCLUSIVE PROPERTY
OR PRIVILEGE IS CLAIMED ARE DEFINED AS FOLLOWS:
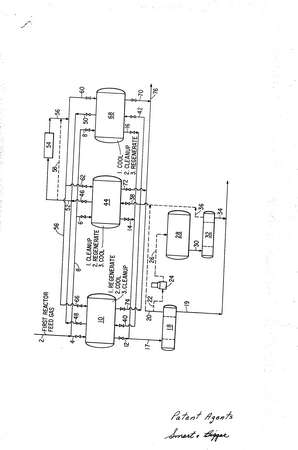
1. In a process for the recovery of free sulfur by reacting a mixture of hydrogen sulfide and sulfur dioxide in a catalytic reaction zone under normal reaction conditions, separating liquid sulfur from the resulting product stream and subsequently discharging into the atmosphere gaseous components from said stream including sulfur compounds, the improvement which comprises reducing the content of said compounds in the aforesaid discharged stream by introducing said mixture at a temperature of from about 450° to about 600°F into a first catalytic reaction zone having sulfur deposited thereon and wherein the catalyst is regenerated and sulfur is simultaneously formed, withdrawing a gaseous product stream from said first zone and separating product sulfur therefrom, introducing the resulting sulfur-denuded stream containing unreacted hydrogen sulfide and sulfur dioxide into a second catalytic reaction zone at an inlet temperature of from about 200° to about 400°F whereby free sulfur is deposited on the surface of the catalyst in said second zone, continuing the deposition of sulfur on said catalyst in said second zone until the amount of hydrogen sulfide and sulfur dioxide in the second zone effluent approaches a preset allowable limit, thereafter changing the flow of said mixture from said first zone to said second zone, employing in the latter an inlet temperature of from about 450° to about 600°F, whereby the catalyst in said second zone is regenerated and sulfur is simultaneously formed therein, removing product sulfur from said second zone effluent, thereafter introducing the resulting sulfur-denuded second zone effluent containing hydrogen sulfide and sulfur dioxide at an inlet temperature of from about 200° to about 400°F into said first zone whereby free sulfur is deposited on the surface of the catalyst in said first zone, continuing the deposition of sulfur on said catalyst in said first zone until the amount of hydrogen sulfide and sulfur dioxide in the said first zone effluent approaches a preset allowable limit, and thereafter repeating the above cycle.
2. The process of Claim 1 wherein the step of depositing sulfur on the catalyst is carried out by introducing said sulfur denuded stream in one direction through said second reaction zone and wherein the sulfur thus deposited on said catalyst is removed therefrom by introducing said 450° to 600°F mixture into said second reaction zone in a direction opposite that in which said sulfur denuded stream was introduced.
3. The process of Claim 2 wherein the step of depositing sulfur on the catalyst is carried out by introducing said sulfur-denuded stream upwardly through said second reaction zone, and wherein the sulfur thus deposited on said catalyst is removed therefrom by introducing said 450° to 600°F mixture in the top of said second reaction zone in downward flow.
4. The process of Claim 2 wherein the regenerated catalyst in said first reaction zone is reduced in temperature by cooling a portion of the effluent from said first reaction zone and combining the resulting cooled portion with said sulfur-denuded stream prior to contact with the hot regenerated catalyst in said first rea.ction zone and continuing said cooling step until the hydrogen sulfide and sulfur dioxide content of said sulfur-denuded stream exceeds a predetermined level.
5. The process of Claim 3 wherein the regenerated catalyst in said first reaction zone is reduced in temperature by cooling a portion of the effluent from said first reaction zone and combining the resulting cooled portion with said sulfur-denuded stream prior to contact with the hot regenerated catalyst in said first reaction zone and continuing said cooling step until the hydrogen sulfide and sulfur dioxide content of said sulfur-denuded stream exceeds a predetermined level.
6. The process of Claim 2 wherein the regenerated catalyst in said first reaction zone is reduced in temperature by cooling a portion of the effluent from said first reaction zone and combining the resulting cooled portion with said sulfur-denuded stream prior to contact with the hot regenerated catalyst in said first reaction zone and continuing said cooling step for a time no longer than that required for the hydrogen sulfide and sulfur dioxide content of said sulfur-denuded stream to exceed a predetermined level.
7. The process of Claim 3 wherein the regenerated catalyst in said first reaction zone is reduced in temperature by cooling a portion of the effluent from said first reaction zone and combining the resulting cooled portion with said sulfur-denuded stream prior to contact with the hot regenerated catalyst in said first reaction zone and continuing said cooling step for a time no longer than that required for the hydrogen sulfide and sulfur dioxide content of said sulfur-denuded stream to exceed a predetermined level.
8. The process of Claim 1 wherein the temperature of the sulfur-denuded stream from said first reaction zone -- in which catalyst regeneration and sulfur formation are occurring -- is adjusted to a value of from about 350° to about 450°F, thereafter introducing said stream into an auxiliary catalytic reaction zone to convert a portion of said hydrogen sulfide and sulfur dioxide into free sulfur, separating the sulfur thus formed, and thereafter effecting said sulfur deposition step in said second zone.
9. The process of Claim 2 wherein the temperature of the sulfur-denuded stream from said first reaction zone in which catalyst regeneration and sulfur formation are occurring is adjusted to a value of from about 350° to about 450°F, thereafter introducing said stream into an auxiliary catalytic reaction zone to convert a portion of said hydrogen sulfide and sulfur dioxide into free sulfur, separating the sulfur thus formed, and thereafter effecting said sulfur deposition step in said second zone.
10. The process of Claim 3 wherein the temperature of the sulfur-denuded stream from said first reaction zone in which catalyst regeneration and sulfur formation are occurring is adjusted to a value of from about 350° to about 450°F, thereafter introducing said stream into an auxiliary catalytic reaction zone to convert a portion of said hydrogen sulfide and sulfur dioxide into free sulfur, separating the sulfur thus formed, and thereafter effecting said sulfur deposition step in said second zone.
11. The process of Claim 1 wherein the combined hydrogen sulfide and sulfur dioxide content of the sulfur-denuded stream from said first reaction zone -- in which catalyst regeneration and sulfur formation are occurring -- is in excess of about 2 volume percent, adjusting the temperature of said stream to a value of from about 350° to about 450°F, thereafter introducing said stream into an auxiliary catalytic reaction zone to convert a portion of said hydrogen sulfide and sulfur dioxide into free sulfur, separating the sulfur thus formed, and thereafter effecting said sulfur deposition step in said second zone.
12. The process of Claim 2 wherein the combined hydrogen sulfide and sulfur dioxide content of the sulfur-denuded stream from said first reaction zone -- in which catalyst regeneration and sulfur formation are occw:ring -- is in excess of about 2 volume percent, adjusting the temperature of said stream to a value of from about 350° to about 450°F, there after introducing said stream into an auxiliary catalytic reaction zone to convert a portion of said hydrogen sulfide and sulfur dioxide into free sulfur, separating the sulfur thus formed, and thereafter effecting said sulfur deposition step in said second zone.
13. The process of Claim 1 wherein the effluent from said sulfur deposition step is employed to cool down the catalyst bed in a third catalytic zone to a temperature of from about 200° to about 350°F prior to discharging said effluent into the atmosphere.
14. The process of Claim 2 wherein the effluent from said sulfur deposition step is employed to cool down the catalyst bed in a third catalytic zone to a temperature of from about 200° to about 350°F prior to discharging said effluent into the atmosphere.
|
 Heritage Community Foundation Presentss
Heritage Community Foundation Presentss






